This page provides guidance for clinicians and histopathology on the process for accessing solid tumour whole genome sequencing via East Genomics for those working outside of Cambridge University Hospitals (CUH).
This page is for those ordering tests from outside of CUH. If you are referring within CUH via EPIC separate guidance is available.
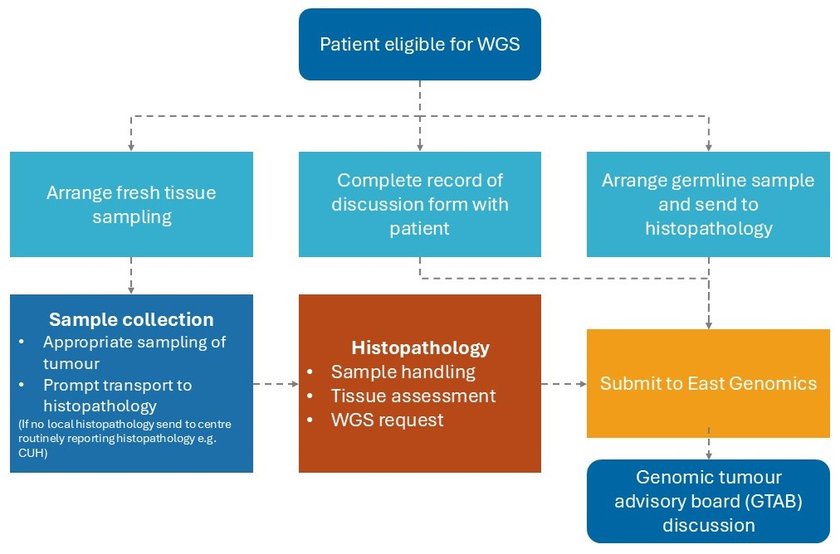
Please ensure you follow all of the below ten steps when ordering WGS.
1. Eligibility for Whole Genome Sequencing (WGS)
2. Consent
3. Tumour samples requirements
4. Collecting samples
The specimen should be collected and submitted either fresh OR in RNAlater (in instances where samples cannot be fast frozen):
5. Handling the specimen in histopathology
6. Germline sample collection
| Patient | Volume of Blood | Blood tube Preservative Type |
|---|---|---|
| Patient Adult | Volume of Blood 3-5ml | Blood tube Preservative Type EDTA |
| Patient Paediatric | Volume of Blood >3ml | Blood tube Preservative Type EDTA |
| Patient Infant | Volume of Blood 1-3ml | Blood tube Preservative Type EDTA |
7. Requesting whole genome sequencing
8. Sending samples for WGS
When sending a sample, please let us know the patient details, and what sample (germline or tumour) is being sent, and details of the courier by e-mail (with subject including “Cancer WGS”).
Please send the 1/ tumour sample, 2/germline sample, 3/ request form, 4/ Record of Discussion (if not already sent electronically) and 5/ a copy of the related histopathology report if available to East Genomics:
East Genomics Laboratory,
Box 143, Level 6 Addenbrooke's Treatment Centre,
Cambridge University Hospital NHS Foundation Trust,
Hills Road, Cambridge, CB2 0QQ
Frozen samples must be transported on dry ice.
Samples that have been collected in and immediately fixed in RNAlater can be transported chilled or at ambient temperature.
Blood samples
Blood samples should ideally be sent at 4oC but it is acceptable to send samples at ambient temperature if the total time from collection to extraction does not exceed 72 hours.
9. Checklist for submitters
- Test request form (cancer WGS-specific form)
- The GLH is unable to order WGS if mandatory information is missing
- Record of Discussion form (WGS-specific)
- Data cannot be returned to East Genomics without a completed and signed Record of Discussion
- Germline sample
- Peripheral blood in EDTA tube, transport chilled (not frozen)
- Tumour sample
- Fresh-frozen or RNAlater
10. Report and discussion at genomic tumour advisory board (GTAB) meeting
Appendix – auto-populated test request form
Go to NHS England test selection page (opens in a new tab).
Search and select relevant clinical indication
If patient is eligible, click Yes, start referral
Click Use the PDF order form
Search and select site requesting WGS (sites served by the East-GLH are listed here.
Continue and then Download order form
Complete form. Mandatory information must be provided (highlighted in screenshot below).
The GLH cannot order WGS without it:
- Requesting organisation (auto populated)
- Patient demographics
- Test directory clinical indication code (auto populated)
- Presentation status
- Solid tumour requests
- Indicate if Primary, Metastatic, Unknown, Lymphoma
- Histopathology Lab ID
- Date of the Diagnosis
- Indicate tumour/germline sample type
- Tumour purity
- Submitter contact details
Print form and send with sample to the East Genomics address indicated on the GMS page.