This page provides guidance for clinicians and histopathology on the process for accessing solid tumour whole genome sequencing via East Genomics for those ordering tests at Cambridge University Hospitals (CUH).
This page is for those at CUH using EPIC. If you are ordering from other Trusts within the East Genomics region separate guidance is available.
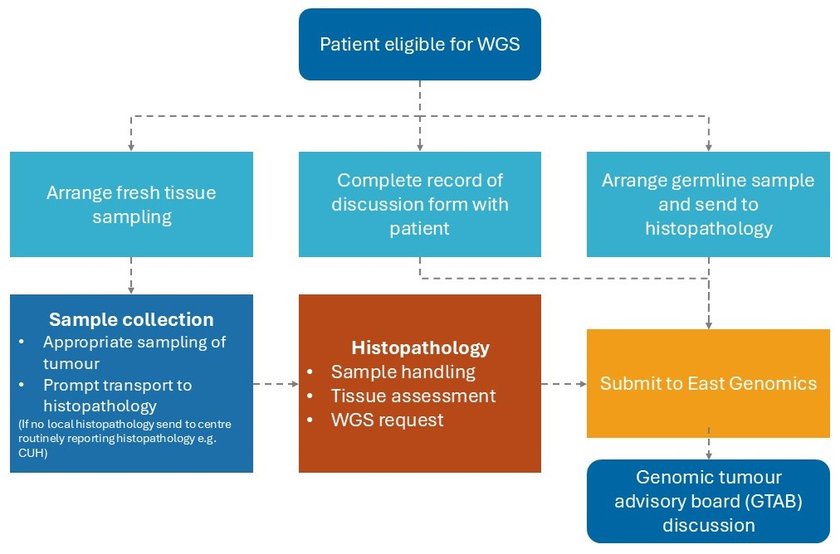
Please ensure you follow all of the below five steps when ordering WGS.
| Patient | Volume of Blood | Blood tube Preservative Type |
|---|---|---|
| Patient Adult | Volume of Blood 3-5ml | Blood tube Preservative Type EDTA |
| Patient Paediatric | Volume of Blood >3ml | Blood tube Preservative Type EDTA |
| Patient Infant | Volume of Blood 1-3ml | Blood tube Preservative Type EDTA |
Appendix 1: How to request the germline bloods in EPIC
GERMLINE BLOOD SAMPLES
The germline sample can be taken as an outpatient or inpatient.
Both the encounter type used, and the procedure version are key. If a germline specimen is not being collected during the inpatient encounter, then an outpatient encounter must be used e.g., Orders Only, and the outpatient version of the procedure/order must be used.
- Please place an order for Solid Cancer WGS Germline samples on EPIC via “Order Entry” > “Enter patient details” > “New”.
- In the pop-up box requiring “Type”, please write “Orders Only” then select “ADD ORDER” on the chart review page which appears.
- Type the Lab-code “LAB8591” for a Solid WGS Germline order.
- Please fill in all the details requested on EPIC with the symbol then sign orders.
- Go to Order enquiry > by patient (enter patient MRN)> Select your order and collect specimens> print labels.
- Please have one of your team take 5ml blood into an EDTA bottle and stick the EPIC labels on the EDTA tube.
For the Germline sample, please have the EDTA tube(s) portered to the GLH – for clarity, the address is below.
FAO: Solid cancer WGS team
East Genomic Laboratory Hub,
Box 143, Level 6 Addenbrooke's Treatment Centre,
Cambridge University Hospital NHS Foundation Trust,
Hills Road,
Cambridge, CB2 0QQ
It is important to ensure that the correct orders are made, so the GLH have visibility of the sample and receive it via our pathway.
Appendix 2: Process of drive through/local blood collection approach
Instructions for clinical team
- Place CANCER WGS GERMLINE order on Epic (via CUH)
- Print test request form via:
- Chart review (labs) > ‘Solid Cancer WGS Germline Sample’
- Order Requisition link (at bottom of WGS order)
- Tick DNA Tests: EDTA (FBC) tube box.

4. Print Epic barcode label
5. Send both test request form + EPIC Label + EDTA tube to patient
6. Patient has blood taken at local hospital
7. Local hospital sends blood + test request form to GLH FAO: Solid cancer WGS team (Address/instructions/collection record all on test request form)