This page provides step-by-step guidance for ordering whole genome sequencing. These tests are ones where Test Method in the National Genomic Test Directory is listed as “WGS”.
For clarification of any of the information on this page please email cuh.eastglh-rarediseases-wgs@nhs.net
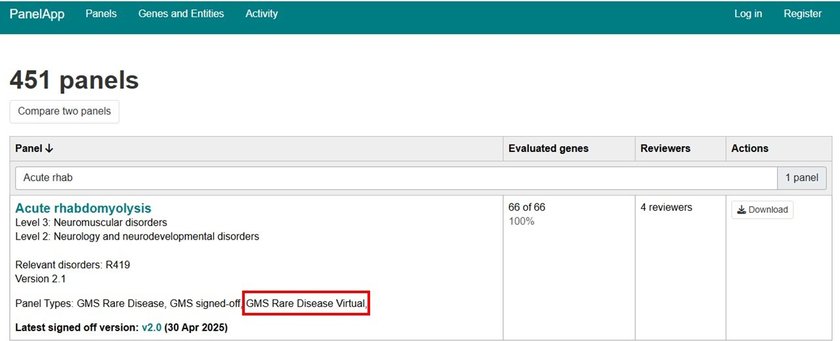
1. Find the test
Please consult the national genomic test directory eligibility criteria document carefully when ordering tests. Make a note of the Test ID for the test you require. For whole genome sequencing tests the Test Method will be “WGS”.
Ensure that your patient meets the eligibility criteria and that your specialism is able to order that test.
2. Patient information and consent
You must have completed training on patient choice and consent via the East Genomics learning platform. (opens in a new tab)
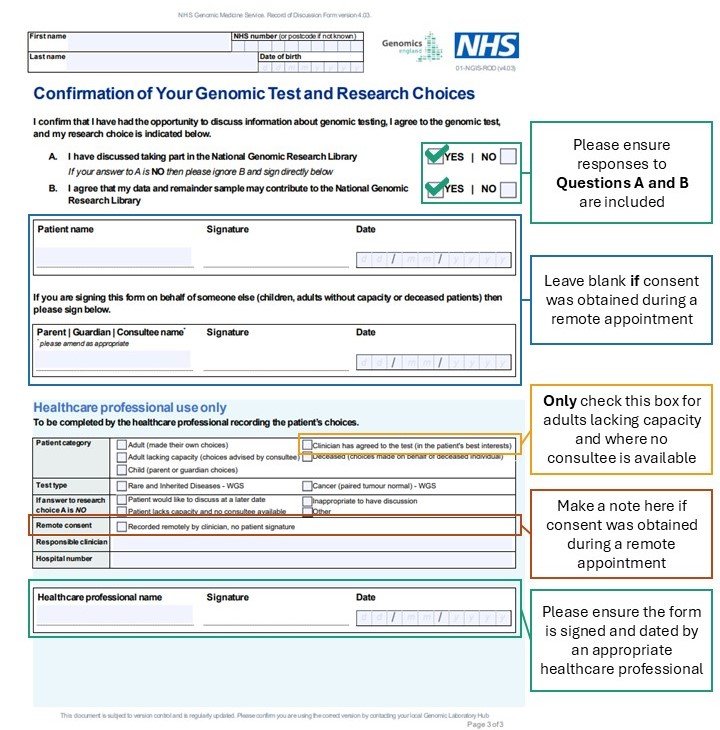
Ensure you have completed a suitable discussion about genomic testing with your patient before ordering testing. Discussions can be performed remotely without the need for a patient signature. There needs to be a record of the discussion retained in the patient record. The discussion must include possible implications for the patient and their family members.
Record of discussion forms are provided alongside our order forms.
Any individuals receiving WGS testing can also be invited to join the National Genomic Research Library.
Find out more about discussing testing with patients and available training to support you.
3. Familial analysis for inherited disorders
Whole genome sequencing is more likely to yield valuable diagnostic insights if performed as a trio (child and both parents) so that comparisons can be made between the three related genomes. Wherever possible please include parental samples in addition to the patient sample.
In some circumstances parental samples may not be available, in which case duos or singletons can be submitted. Please contact the team if you wish to submit multiple affected individuals per family or additional family samples.
You must have a discussion about genetic testing with every individual tested and a separate record of discussion form must be completed for each one.
4. Order forms
Download whole genome sequencing forms, record of discussion forms and patient information leaflets
- One order form is required per proband (affected patient) – separate forms are not needed for parental samples.
- Use the trio test order form when submitting a WGS test that includes parental samples.
- One record of discussion form is required for each person tested – separate forms are needed for each parent and any additional family members
The order form and record of discussion must be sent with the sample or to cuh.eastglh-rarediseases-wgs@nhs.net.
5. Collecting and sending samples
If a suitable sample has not already been collected from your patient and stored you will need to send us a sample for analysis.
6. Results
Results are sent via email to the provided email address.
We encourage you to use a pool email rather than your individual contact information.
We make every effort to return results and meaningful analysis within target turnaround times. Depending on the test this can vary between a few days and several months. Visit our turnaround times page to see what these targets are and our recent performance statistics.